The Good the Bad and the Ugly of Inflamation
.Are you suffering from any of the 5 cardinal signs ? Redness, Swelling, Heat, Pain and or loss of function? These are all danger signs As inflammation progresses, however, it begins to damage your arteries, organs and joints. Left unchecked, it can contribute to chronic diseases, such as heart disease, blood vessel disease, diabetes, obesity, cancer, Alzheimer's disease and other conditions.
According to a recent article from Vanderbilt Medicine Magazine - “Inflammation is the body’s response to microbial, autoimmune, metabolic or physical insults,” including burns and physical trauma, said Hawiger, Distinguished Professor of Medicine and Louise B. McGavock Professor.
White blood cells, including granulocytes and macrophages, are the “first responders” to sites of infection and injury. They emit waves of chemicals that can kill germs outright, and protein messengers called cytokines to carry out a bewilderingly wide array of duties.
When these weapons misfire, however, they can wreak havoc. They can even kill.
TWIN PEAKS
Obesity and its constant companion, type 2 diabetes, are at epidemic proportions in this country. One thing that connects them is inflammation.
Normally, the first responders to the site of injury or infection are white blood cells—including macrophages. They produce waves of chemicals, including cytokines, which can kill germs and sound the alarm for other populations of inflammatory cells.
But fat cells can produce cytokines, too. And as fat tissue grows, it attracts inflammatory cells, particularly macrophages. The burden of obesity also crushes fat cells to death. And that makes the problem worse, as inflammatory cells move in to clean up the debris.
Inflammation also antagonizes the action of insulin, the hormone that stimulates muscle and liver to absorb glucose from the blood. And obesity, insulin resistance and type 2 diabetes, in turn, increase the risk for heart disease.
During the past three years, Vanderbilt researchers Lan Wu, M.D., and Luc Van Kaer, Ph.D., Elizabeth and John Shapiro Professor, have identified two subpopulations of white blood cells that play key roles in obesity-associated inflammation.
One group of white blood cells, invariant natural killer lymphocytes, overproduces inflammatory cytokines that worsen insulin resistance. Another group, innate-like regulatory B lymphocytes, secretes an anti-inflammatory cytokine, interleukin-10 (IL-10).
Normally the forces of inflammation are held in delicate balance. But obesity “tilts” it in favor of inflammatory cytokines.
Wu and Kaer restored balance in an animal model by isolating regulatory B lymphocytes from lean mice and transferring them into obese ones. The cells made their way to the fat tissue and released IL-10, which improved insulin sensitivity.
This suggests that a regulatory B cell-based “therapy” could interrupt obesity-related inflammation and reverse insulin resistance.
“There is still a long way to go,” cautioned Wu, research associate professor of Pathology, Microbiology and Immunology. “You cannot design effective therapies without knowing what comes in, what goes out and ‘what’s cooking’ in the middle.”
HEART DISEASE RISK
LDL (low-density lipoprotein), the “bad” form of cholesterol, has long been considered a major risk factor for heart disease, while HDL (high-density lipoprotein), the “good” cholesterol, is thought to have a protective effect.
MacRae Linton, M.D., is studying the role of the macrophage in atherosclerosis. Photo by John Russell.
But some people have heart attacks and strokes even though their LDL levels are normal and their HDL levels are high. Again, inflammation may be involved here. While HDL’s main job is to transport excess cholesterol to the liver for disposal, it also is anti-inflammatory, anti-oxidant, and anti-thrombotic.
Problems in any of these areas “may contribute to that residual (heart disease) risk,” said MacRae F. Linton, M.D., the Dr. Stephen Schillig Jr. and Mary Schillig Professor of Medicine and Professor of Pharmacology. Linton is leading a major project at Vanderbilt supported by a five-year, $11.8 million federal grant to search for markers of HDL “dysfunction.”
Recently Vickers and his colleagues reported that a tiny piece of RNA called microRNA-223 is a “master regulator” of cholesterol production and transport in the liver.
Because microRNA-223 is also involved in regulating inflammation, it “provides an interface between inflammation and cholesterol,” and thus is a potential drug target, said Vickers, assistant professor of Medicine.
SNEAKY DEVILS
To mount an effective response against a foreign invader, white blood cells must proliferate rapidly. They’re “turned on” when they bind to inflammatory cytokines, notably interleukin 4 and interleukin 13. Overproduction of IL-4 and IL-13, however, can trigger an asthma attack.
Turns out certain cancers, particularly of the colon and breast, have “hijacked” inflammation’s proliferative potential. Unlike normal tissues, they have, through natural selection, acquired the ability to express IL-4 and IL-13 receptors.
When the cytokines bind, they trigger rapid tumor growth. They also enable tumor cells to travel to distant corners of the body and survive the journey, a process known as metastasis. “These molecules … actually can change how tumor cells behave,” says Barbara Fingleton, Ph.D., assistant professor of Cancer Biology.
Asthma drugs that block IL-4 and IL-13 are in clinical development, but their effects are too broad to be used to fight cancer. So Fingleton and her colleagues are identifying special characteristics of the tumor receptors that could be targeted by new drugs specific for tumor cells.
BRAIN TARGET
Sometimes inflammation flares up even before birth.
Animal and human studies suggest that “maternal immune activation” in response to infection during pregnancy can alter the development of the fetal brain in ways that increase the risk for schizophrenia and autism in the offspring.
The culprit is not the germ but the inflammatory cytokines released in response to it, notably interleukin 6 (IL-6), says Karoly Mirnics, M.D., Ph.D., James G. Blakemore Professor of Psychiatry.
Mirnics and his colleagues were the first to describe, in postmortem studies, immune disturbances in the brains of people with schizophrenia and autism. In studies in mice, they showed that IL-6 is one of the critical mediators in this inflammatory pathway of brain disturbance.
These studies one day may lead to a better understanding of the neurotoxic processes that occur in the developing brain, and of ways to protect it.
It won’t be easy.
“The interplay of genetics and environment … is really an incredibly complex thing,” Mirnics says, “because it occurs on a developmental timeline … your susceptibility to different factors changes.”
One thing is certain: mothers are not at fault. “You cannot avoid contact with bugs,” he says.
The best way to protect the fetus is not to avoid human contact during pregnancy but to get a flu shot. Compared to full-blown flu, vaccination produces “minimal” immune activation that is unlikely to increase risk for later disease, Mirnics says.
Inflammation can strike any part of the body. In the visual system, astrocyte glial cells normally protect the optic nerve and retina. But when these cells become “reactive” in response to injury or diseases like glaucoma, they can proliferate, enlarge and over-produce cytokines to the point that the optic nerve and retina are destroyed.
To blunt this response, David Calkins, Ph.D., and his colleagues are testing a class of drugs used to treat rheumatoid arthritis. When given in the form of eye drops in an animal model of glaucoma, these drugs, called p38 MAP kinase inhibitors, protected the optic nerve.
Many diseases have “in common a very potent inflammatory component,” said Calkins, the Denis M. O’Day Professor of Ophthalmology and Visual Sciences. “The potential for cross-disciplinary application of drugs is tremendous. We really need to explore this.”
HARD BARGAIN
Today’s headlines may be filled with Ebola, but 30 years ago the human immunodeficiency virus (HIV) was the biggest viral menace on the planet. Today acquired immune deficiency syndrome (AIDS) is a manageable disease, thanks to the development of effective anti-viral drugs.
But as survival has increased, so have “co-morbid” conditions including diabetes, and heart and kidney disease that appear to be related to chronic infection. “That inflammation persists even after you reduce the amount of virus in the blood to undetectable levels,” said John Koethe, M.D., assistant professor of Medicine.
The same goes for the brain, said Todd Hulgan, M.D., MPH, associate professor of Medicine who is investigating the connection between chronic neuroinflammation and HIV-associated neurocognitive disorders as part of a national study group.
“In many ways HIV becomes a model of a chronic inflammation,” Hulgan said. Learning what’s happening here could advance understanding of other inflammation-associated diseases as well, he added.
BLOOD ATTACK
One of the most graphic examples of out-of-control inflammation is sepsis, a systemic inflammatory response to infection that eventually undermines the delicate microcirculatory meshwork supplying oxygen and nutrients to the vital organs.
Jacek Hawiger, M.D., Ph.D., estimates that 80 percent of all major human diseases are mediated by inflammation. Photo by John Russell.
Often called blood “poisoning,” it’s actually a fast-and-furious “blood attack” Hawiger said.
Sepsis causes endothelial cells lining blood vessels to leak, disrupting circulation. In severe cases, it can lead to acute septic shock, multiple organ failure and rapid death. In fact, sepsis is the third leading disease killer in the United States, after heart attacks and cancer, he said.
But it is not invulnerable.
One approach to stopping sepsis is to intervene in the cascade of events that leads to it. A paradigm of inflammation conceived and tested in Hawiger’s laboratory led to the design of a new class of anti-inflammatory peptides named “nuclear transport modifiers.”
These cell-penetrating peptides increased survival in mice exposed to high doses of a bacterial endotoxin by preventing expression of inflammatory genes.
Recently, Hawiger and his colleagues demonstrated in animal and human cells lining blood vessels a new anti-inflammatory “rheostat” called CRADD, a protein that puts on brakes of excessive inflammatory signaling to the nucleus.
These discoveries may assist development of new therapies for inflammatory vascular disorders that disrupt the “integrity” of the endothelial lining in lungs, liver, kidneys, heart and brain.
In one sense, sepsis is a “genomic storm,” Hawiger said, a titanic struggle between factors turned on by the invading microbial genome and the defending immune and inflammatory responses activated by the “command center” of the host genome, the nucleus of the cell.
This war will be won, he asserted, when scientists learn how to “tune” the defensive signals in a way that turns up the “good” of inflammation and turns down the “bad.”
Detailed eh? so, How can we help?
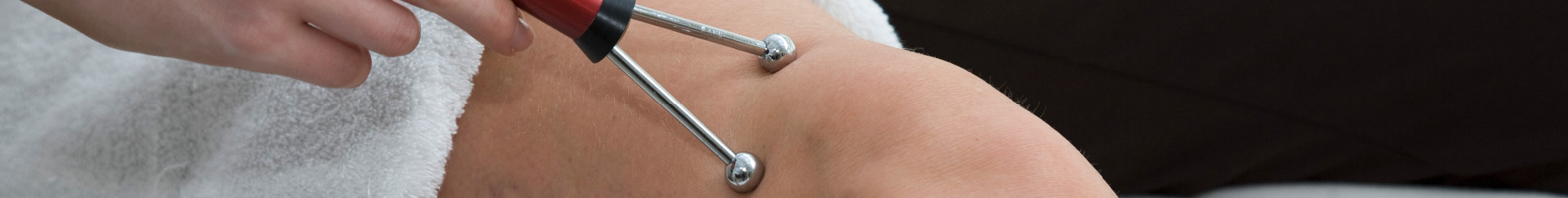
Using our technology we treat the area affected for just under an hour.
Pain, inflammation and muscle spasms often result when muscles, ligaments or tendons are injured. The circulation in the injured areas is effected adversely. There is an accumulation of lactic acid and other waste products in the tissues. This slows the healing process.
Detailed eh? so, How can we help?
The Acuscope and Myopulse simultaneously measure and balance the abnormal electrical resistance in injured tissue. The electrical conductance of the injured tissue is examined and compared to a preprogrammed parameters for healthy tissue. When electrical conductance is outside the normal, healthy range, the instruments change the wave form, frequency, intensity and polarity to restore the tissue integrity to promote healing.
The Myopulse eliminates inflammation; the Acuscope promotes healing in joints to promote the growth of cartilage and synovial fluids.
There is little or no sensation because micro-current energy is similar to the energy inside the body. Micro-currents are one-millionth the strength of household current. They are compatible with the body’s bio electrical communication system and support the self-healing feedback mechanism already present at the cellular level. The effects of micro-currents on the healing process has been documented in the scientific literature for many years.
When this energy is introduced into the cells, circulation, lymphatic drainage, waste product removal, cellular metabolism improve. The flow of other forms of biological energy similar to chi or vital force is accelerated. Acidic waste products are flushed from the tissues and the body’s healing powers are accentuated. This results in an accelerated healing response at the cellular level, leading to reduction of pain and improved
Would you like to know more? Call Sue on 07920770606 or email@advancedhealingtherapies.co.uk

Share this post: